
The 8HUM Model
Exploiting the 8HUM mouse,
a unique mouse model with multiple utilities in drug discovery, and development
Drug-drug interactions are increasingly recognised as a major health care problem. Extra health care costs caused by unwanted DDIs run into the $100s billions per year. In addition unwanted DDIs can cause immense suffering to patients, as measured by increased morbidity and mortality.
As populations age, and take more drugs, and as treatments for diseases become more complex, involving multiple drugs, the problem of DDIs will only increase.
Because the majority of DDIs are caused by interactions with the CYP450 system, and because rodent CYP450s metabolise compounds in a different way to human CYP450s, there has been no way of accurately measuring DDIs in a pre clinical setting.
The 8HUM model changes this, because it is able to metabolise drugs in a human-like way. It provides human like pharmacokinetics and human like metabolites.
Therefore, uniquely, 8HUM can be used to predict DDIs in a pre clinical test. Most importantly it is able to predict DDIs for multiple drugs delivered at the same time (polypharmacology). No other pre-clinical model is able to provide these type of data.
This property of 8HUM makes it uniquely valuable in being able to provide data on DDIs for compounds already on the market.
8HUM is also very valuable in being able to guide clinical trials of new drugs to:
- Optimise drug development with respect to DDI
- Provide guidance for optimal development of combination therapies in disease
PhaSER BIOMEDICAL
The DDI Analyser Vision
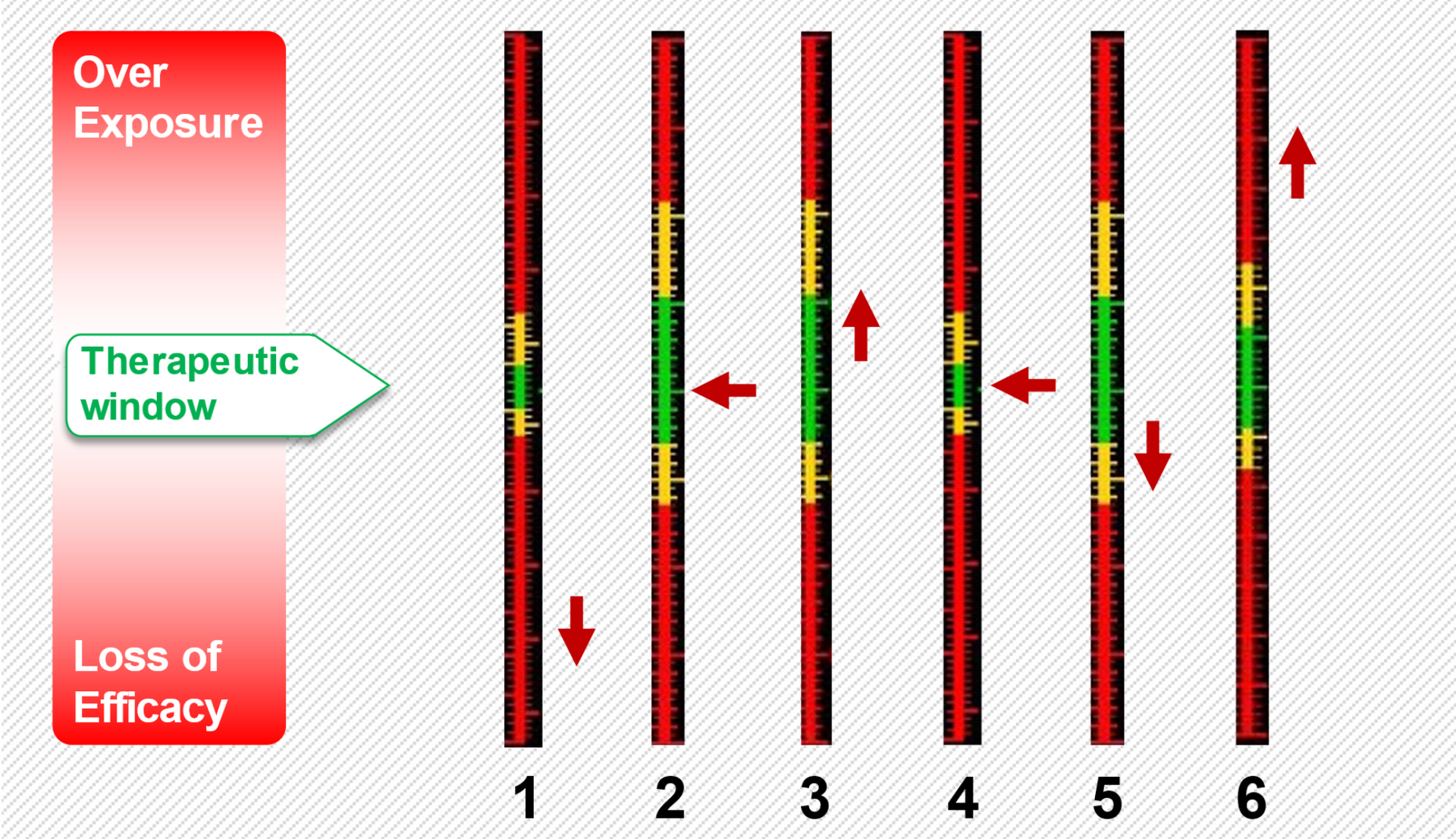
Drugs levels when administered in Polypharmacy regimen against therapeutic window.
Figurative image on how the DDI analyser will work. Areas in green represent the safe, efficacious level of the drugs being tested (1-6) . Areas above the green bar represents overexposure/side effects and below represents loss of efficacy. The user will enter the drugs being prescribed and the dose and the adviser will indicate whether there are risks of a DDI. When a new drug is added to the regimen the adviser will indicate there is a new risk of a DDI for the new drug combination. The analyser will then suggest a change in drug dose or an alternative drug to optimise the therapy so that all the
arrows become aligned in the green areas .